The Fundamentals of Mixer Settler Technology in Pharmaceutical Purification
Operating Principles of Mixer Settlers
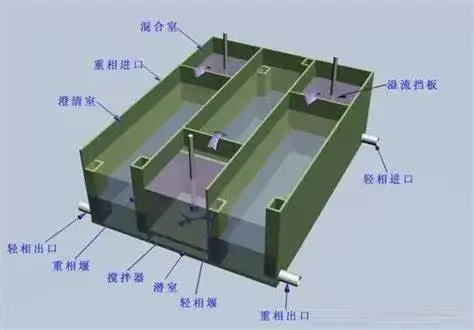
Mixer settlers operate on the principle of liquid-liquid extraction, utilizing the difference in solubility between two immiscible phases to separate compounds. The process begins in the mixing chamber, where an aqueous phase containing the target compound is vigorously mixed with an organic solvent. This agitation promotes mass transfer, allowing the desired molecules to migrate into the organic phase. The mixture then flows into the settling chamber, where gravity separation occurs. The heavier aqueous phase settles to the bottom, while the lighter organic phase, now enriched with the extracted compound, rises to the top. This separation is facilitated by carefully designed baffles and weirs that guide the flow and prevent remixing.
Key Components and Design Features
Modern mixer settlers boast several essential components that enhance their performance in pharmaceutical purification. The mixing chamber typically features a high-efficiency impeller designed to create optimal turbulence for thorough phase contact. The settling chamber incorporates strategically placed baffles to promote smooth flow and minimize turbulence, ensuring clean phase separation. Advanced models may include integrated sensors for real-time monitoring of pH, temperature, and interface levels, allowing precise control over the extraction process. Materials of construction are carefully selected to withstand aggressive solvents and maintain product purity, with options including polypropylene (PP), polytetrafluoroethylene (PTFE), and high-grade stainless steel (SUS316L) for corrosion resistance.
Advantages in Pharmaceutical Applications
Mixer settlers offer numerous advantages that make them particularly suitable for pharmaceutical purification. Their modular design allows for easy scaling and customization to meet specific process requirements. The ability to adjust parameters such as mixing intensity, residence time, and phase ratios provides flexibility in handling various compounds and achieving desired purity levels. Compared to other separation techniques, mixer settlers often require lower capital investment and operational costs while delivering high extraction efficiency. Additionally, their continuous operation capability aligns well with the pharmaceutical industry's demands for consistent quality and high-volume production. The closed-system design also minimizes solvent loss and exposure, contributing to both cost-effectiveness and environmental safety.

Specific Applications in Pharmaceutical Purification Processes
API Extraction and Purification
In the realm of active pharmaceutical ingredient (API) production, mixer settlers excel at extracting and purifying valuable compounds from complex reaction mixtures. For instance, in the synthesis of antibiotics, these systems can effectively separate the desired antibiotic molecule from byproducts and unreacted starting materials. The process typically involves multiple extraction stages, with each stage incrementally increasing the purity of the API. By carefully selecting solvents and optimizing extraction conditions, pharmaceutical manufacturers can achieve high yields and purity levels that meet stringent regulatory standards. This application of mixer settlers not only enhances product quality but also streamlines downstream processing steps, reducing overall production costs.
Chiral Separation for Enantiomeric Purity
Chiral separation is a critical process in pharmaceutical manufacturing, particularly for drugs where one enantiomer is therapeutically active while the other may be inactive or even harmful. Mixer settlers equipped with chiral selectors or coupled with chiral chromatography techniques offer an efficient solution for large-scale enantiomeric purification. The process involves creating a two-phase system where one phase contains a chiral selector that preferentially binds to one enantiomer. As the mixture passes through the mixer settler stages, the desired enantiomer is progressively extracted, resulting in a high-purity product. This application is particularly valuable in producing single-enantiomer drugs, which often exhibit improved efficacy and reduced side effects compared to their racemic counterparts.
Impurity Removal and Final Product Polishing
In the final stages of pharmaceutical production, mixer settlers play a crucial role in removing trace impurities and polishing the product to meet pharmacopeia standards. This process often involves liquid-liquid extraction using carefully selected solvents that selectively remove specific impurities while leaving the desired compound in the product stream. Multiple mixer settler stages may be employed in series, each targeting different classes of impurities. For example, in the production of peptide-based drugs, mixer settlers can effectively remove residual protecting groups, unreacted amino acids, and synthesis byproducts. The ability to fine-tune extraction parameters allows manufacturers to achieve impurity profiles well below regulatory limits, ensuring product safety and efficacy.

Optimizing Mixer Settler Performance for Pharmaceutical Purification
Process Parameter Optimization
Achieving optimal performance in pharmaceutical purification requires careful tuning of mixer settler parameters. Key variables include mixing intensity, phase ratio, and residence time in both mixing and settling chambers. Mixing intensity, typically controlled by impeller speed, must be sufficient to ensure thorough phase contact without causing emulsification that could hinder separation. The phase ratio, or the relative volumes of aqueous and organic phases, directly impacts extraction efficiency and selectivity. Residence time affects the degree of mass transfer and separation, with longer times generally improving purity but potentially reducing throughput. Advanced process control systems, incorporating real-time monitoring and feedback loops, enable continuous optimization of these parameters to maintain peak performance even as feed composition or process conditions fluctuate.
Solvent Selection and Recycling Strategies
Selecting the appropriate solvent system is crucial for effective pharmaceutical purification using mixer settlers. Ideal solvents exhibit high selectivity for the target compound, good phase separation characteristics, and compatibility with downstream processes. In many cases, a combination of solvents may be used to achieve the desired separation. Environmental and safety considerations also play a significant role in solvent selection, with an increasing emphasis on green chemistry principles. To enhance sustainability and reduce costs, many pharmaceutical manufacturers implement solvent recycling strategies. These may involve distillation or membrane-based separation techniques to recover and purify spent solvents for reuse in subsequent extraction cycles. Effective solvent management not only improves process economics but also aligns with regulatory requirements for environmental stewardship.
Scale-up Considerations and Process Integration
Scaling up mixer settler operations from laboratory to industrial scale requires careful consideration of hydrodynamics, mass transfer kinetics, and equipment design. Computational fluid dynamics (CFD) modeling can provide valuable insights into flow patterns and mixing behavior at larger scales, guiding equipment design and operating parameters. As scale increases, maintaining uniform mixing and efficient phase separation becomes more challenging, often necessitating modifications to impeller design or the addition of internals in the settling chamber. Integration of mixer settlers into broader pharmaceutical production processes demands attention to material compatibility, clean-in-place (CIP) requirements, and process analytical technology (PAT) implementation. By addressing these scale-up and integration challenges, pharmaceutical manufacturers can leverage the full potential of mixer settlers to achieve high-purity products with consistent quality across production batches.
Conclusion
Mixer settlers have proven to be invaluable tools in pharmaceutical purification, offering a versatile and efficient means of separating and purifying complex mixtures. Their ability to handle continuous operations, coupled with precise control over extraction parameters, makes them well-suited for a wide range of applications in API production, chiral separations, and impurity removal. As the pharmaceutical industry continues to evolve, with increasing demands for higher purity standards and more efficient processes, the role of mixer settlers is likely to expand further. By optimizing their performance through careful parameter control, solvent selection, and strategic process integration, pharmaceutical manufacturers can leverage these systems to enhance product quality, improve production efficiency, and meet stringent regulatory requirements.
Contact Us
Ready to elevate your pharmaceutical purification processes? Cuiyan Technology offers state-of-the-art mixer settler solutions tailored to your specific needs. Our expert team can help you optimize your extraction processes, improve product purity, and boost production efficiency. Contact us today at wangzhijun@cuiyan-tec.com to discover how our innovative technologies can transform your pharmaceutical manufacturing operations.